Successfully conducting clinical evaluation of medical devices: from the PICOS method to NOÉ
4 juil. 2025
5 min read
Picos
The PICOS method
The PICOS method
The rigor of a literature review is based on a well-defined research protocol, which clearly describes the context, objectives, research question, as well as the methods for identifying, selecting, and evaluating relevant publications.
Whether conducted as part of a clinical evaluation or a synthesis intended to guide healthcare decision-making, article selection must be objective, systematic, and justified — including all relevant data, whether favorable or unfavorable.
To ensure the scientific quality of this process, MEDDEV 2.7.1 rev. 4 and MDCG 2020-13 recommend the use of structured methodological tools such as the PICOS methodology (Population, Intervention, Comparison, Outcome, Study type), which helps formulate clear and reproducible search strategies.
Commonly used in systematic reviews, PICOS guides the collection of evidence-based data in evidence-based medicine.
In this context, tools such as PICOS play a key role in combining methodological rigor with efficiency in the analysis of scientific literature.
Why is a structured methodology essential?
Without a methodology, a literature review is likely to be biased, incomplete, and non-reproducible. This is why regulatory authorities define a framework for how literature reviews must be conducted: in accordance with Article 61 and Annex XIV of the MDR, research must be systematic, transparent, and objective to avoid arbitrary study selection and to ensure that only relevant evidence is taken into account.
There are four key implications to keep in mind:
Define the research question in advance;
Exhaustiveness: search multiple databases (PubMed, Cochrane, ClinicalTrials.gov, etc.);
Traceability and reproducibility: the entire research methodology must be documented (clear inclusion and exclusion criteria) so that it can be reproduced by another expert or assessor;
Objectivity: critical appraisal of the methodological quality of the identified studies.
What does PICOS mean?
PICOS consists of five components:
P – Population → Identify the patient populations concerned by the device
I – Intervention → Precisely define the intervention or use of the device
C – Comparator → Define a comparator, which may be a device already used in routine practice, no intervention, or possible alternatives
O – Outcome → Specify the expected clinical outcomes
S – Study type
This structure then guides database searches (PubMed, ClinicalTrials.gov, Embase, etc.), keyword selection, and study inclusion/exclusion criteria.
How does it work in practice?
Example: connected insoles for diabetic feet
MONIFEET is a system of connected insoles that monitor plantar temperature.
It is a pair of smart insoles intended for patients whose condition requires regular monitoring of foot temperature. Each insole integrates temperature sensors and an accelerometer (step count), powered by a non-rechargeable battery.
How should the PICOS method be applied to conduct research on this device?
Here is an incomplete research question, which we will refine using the PICOS method:
“Do connected insoles help prevent podiatric complications in people with diabetes?”
→ This question is too vague: which exact population? Which device? Which comparator? Which outcome?
We begin with the “P” of PICOS by defining the population. We can then refine the question as follows:
“In adults with diabetes at risk of diabetic foot ulcers (P), do connected insoles help prevent podiatric complications?”
Next, we must specify the device and, more importantly, the intervention — the “I” of PICOS. The question can then be reformulated as:
“In adults with diabetes at risk of diabetic foot ulcers (P), does continuous plantar temperature monitoring using MONIFEET connected insoles (I) reduce the incidence of plantar ulcers?”
We now have a solid foundation, with a defined target population and intervention that will guide the literature search. We must now add the expected outcomes (“O”) and the comparator (“C”). The final research question becomes:
“In adults with diabetes at risk of diabetic foot ulcers (P), does continuous plantar temperature monitoring using MONIFEET connected insoles (I) reduce the incidence of plantar ulcers over 6 months (O) compared with standard care (visual inspection + occasional infrared thermometer measurement) (C)?”
Each component of the question is now clear and explicit: the question is ready for the literature review. The next step is to identify and organize the keywords that will be used to search scientific databases based on the PICOS framework.
For example, for a PubMed search focused on the population (adults with diabetes at risk of diabetic foot), keywords could include: “Diabetic Foot”, “Diabetes Mellitus”, “Foot Ulcer”, along with synonyms or free-text terms such as “plantar neuropathy”, “high-risk foot”, or “pre-ulcer”.
Next, inclusion and exclusion criteria can be defined. Using our example:
Inclusion criteria: Adults ≥ 18 years with type 1 or type 2 diabetes and at risk of or with a history of diabetic foot
Exclusion criteria: children, adolescents, non-diabetic patients, and post-amputation feet
Based on the elements defined above (Population, Intervention, Comparator, Outcome, Study), NOÉ enables clear and visual structuring of eligibility criteria for a systematic review.
Below is an example of how PICOS fields can be completed in NOÉ for this clinical case:
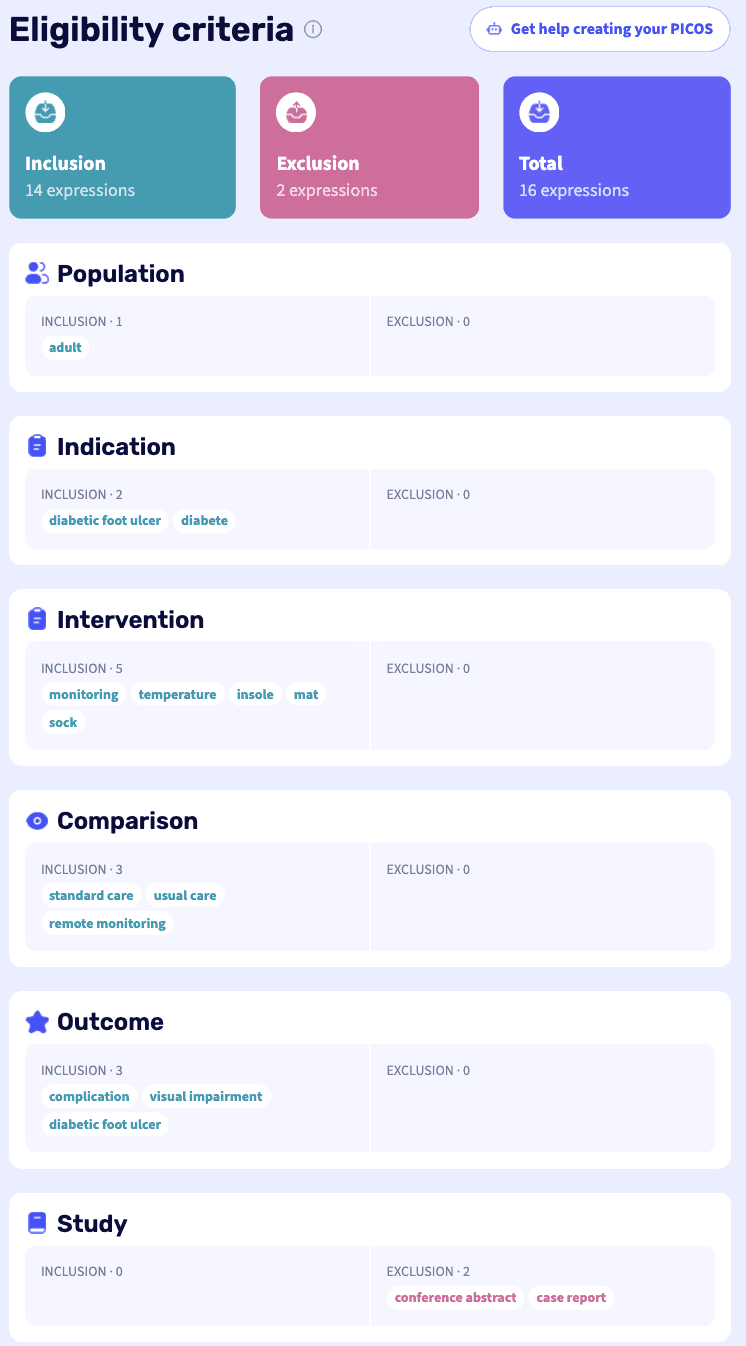
Finally, within the databases, we follow the same approach, as in the previous example for the population, applying the other PICOS criteria (Intervention, Comparator, and primary Outcome).
